Research Area: Immunologia e neuroimmunologia
Group Leader
Immunity, Inflammation & Angiogenesis Laboratory studies signals modulating angiogenesis, inflammation and immunity in various physiopathological conditions.
The Cardiac microenvironment in ACM
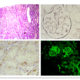
Cardiovascular diseases are the worldwide leading cause of death. Among them, arrhythmogenic cardiomyopathy (ACM) is a major cause of sudden death in the young and in athletes. ACM is a rare (1:2000-1:5000) congenital disease with an autosomal-dominant trait. ACM-causing genes mostly encode major components of the cardiac desmosome and up to 50% of ACM patients harbor mutations in one of them. The structural substrate of ACM consists of progressive myocardial dystrophy with fibro- fatty replacement in the ventricular walls, starting from the subepicardium. Our hypothesis is that mesenchymal stromal cells (MSC) play a crucial role in the disease. Taking advantage of recently established mouse models of ACM, we study the role of MSC and infiltrating cells in the pathogenesis of ACM.
Collaborators: Cristina Basso, Marco Mongillo, Tania Zaglia, University of Padua.
Exploiting MSC-derived EVs to fight cancer
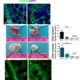
Pathological angiogenesis is a hallmark of several conditions including eye diseases, inflammatory diseases, and cancer. Stromal cells play a crucial role in regulating angiogenesis through the release of soluble factors or direct contact with endothelial cells. We analysed the properties of extracellular vesicles (EVs) released by bone marrow mesenchymal stromal cells (MSCs) and explored the possibility of using them to therapeutically target angiogenesis. We demonstrated (Angioni et al. JEV 2020) that in response to pro-inflammatory cytokines, MSCs produce EVs that are enriched in TIMP-1, CD39 and CD73 and inhibit angiogenesis targeting both extracellular matrix remodelling and endothelial cell migration. Our final goal is to exploit the anti-angiogenic EVs to target pathological tumor associated angiogenesis.
Collaborators: Maurizio Muraca, University of Padua & IRP.
Multiple sclerosis and acquired autoimmune demyelinating syndromes
Acquired demyelinating diseases of the central nervous systems (CNS) constitute a broad spectrum of highly disabling inflammatory and neurodegenerative diseases. Multiple sclerosis (MS) and the so-called Neuromyelits Optica-Spectrum Disorders (NMO-SD), whose incidence and prevalence are dramatically increasing worldwide, may have a pediatric onset, characterized by severe clinical and neuroradiological pictures. The aim of our project is the discovery of still unknown immunopathogenic mechanisms in pedMS through: i) a comprehensive, single-cell multi-omics analysis of the pathogenic cell populations; ii) the identification of possible target antigens, and iii) the identification of novel antigens through T cell receptor (TCR) and HLA profiling.
Collaborators: Paolo Gallo, Stefano Sartori, University of Padua & IRP.
Covid-19: understanding the role of inflammation and immunity
Covid-19 has overwhelmed the sanitary system worldwide. The triggering and tuning of the immune response in patients have immediately come out as a burning issue to be addressed to clinically manage the disease and the patients’ outcome. The identification of key immune signatures and their contribution to SARS-Cov2 infection and pathology represent the forefront of COVID-19 research. Our projects take up this hard challenge by performing a comprehensive analysis of the immune contexture in COVID-19 patients by exploiting novel, state of the art approaches.
Collaborators: Annamaria Cattelan, Giuseppe Testa, Paolo Rossi, Carlo Giaquinto
Group Members
Barbara Molon Assistant Professor
Roberta Angioni PostDoctoral Researcher
Cristina Liboni PostDoctoral Researcher
Chiara Cioccarelli PhD student
Gloria Orlando PhD student
Alessandra Maria Testa PhD student
Fransisca Venegas PhD student
Fabio Munari Lab Technician
Nicole Bertoldi Lab Techinican
Selected Publications
R.L. Contento, S. Campello, A.E. Trovato, E. Magrini, F. Anselmi and A. Viola. 2010. Adhesion shapes T cells for prompt and sustained T cell receptor signaling. EMBO Journal, 29:4035-47.C.
Mazzon, A. Anselmo, J. Cibella, C. Soldani, A. Destro, N. Kim, M. Roncalli, S.J. Burden, M.L. Dustin, A. Sarukhan and A. Viola. 2011. The critical role of agrin in the hematopoietic stem cell niche.. Blood, 118(10): 2733-2742.
Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri PL, Monegal A, Resci-gno M, Savino B, Colombo P, Jonjic N, Pecanic S, Lazzarato L, Fruttero R, Gasco A, Bronte V, Viola A. 2011. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. The Journal of Experimental Medicine 208(10):1949-62.
Kallikourdis M, Trovato AE, Anselmi F, Sarukhan A, Roselli G, Tassone L, Badolato R, Viola A. 2013. The CXCR4 mutations in WHIM syndrome impair the stability of the T cell immunological synapse. Blood 122(5):666-73.
Wang CM, Ploia C, Anselmi F, Sarukhan A, Viola A. 2014. ATP acts as a paracrine signaling molecule to reduce the motility of T cells. EMBO J 33(12):1354-64.
Contatti

Corso Stati Uniti, 4 F
35127 Padova
Phone: +39 049 9640111
Fax: +39 049 9640101
info@irpcds.org
Orario di apertura: lun-ven 8:30 – 17:30